Longread
Ethylene management in practice
Ethylene (or ethene) is a gaseous compound which is always present in the atmosphere. Atmospheric ethylene originates from microbial activity in the soil and water, production by plants, and industry and motor vehicle exhaust. Mostly concentrations are very low and even undetectable. But in the fresh produce supply chain the ethylene can be present in high concentrations. This is linked to the ethylene production of fresh produce itself. Ethylene accumulates in confined spaces during storage, transportation and packaging. In this article, the postharvest consultants of Wageningen Food & Biobased Research provide practical advices.
Relevance to practice
Ethylene is less frequently measured than temperature, humidity, oxygen and carbon dioxide. However, ethylene is present throughout the supply chain and relevant for postharvest management. It is also one of the air conditions that we can control with practical interventions if necessary. The (underestimated) presence of high ethylene can directly affect quality and lead to suboptimal application of practical solutions. When negative effects of ethylene are suspected, it is wise to monitor the concentration and apply preventive strategies.
Ethylene: sometimes favorable, sometimes harmful
Ethylene is also called the ripening hormone. It is very relevant for the ripening of many fruit species. Most fruits produce ethylene to start the ripening process. Its action can be seen as positive when it leads to ripe and tasty products. Ethylene gas is even artificially supplied in some cases to regulate ripening, for example for the ripening of bananas, mangoes and avocados. Ethylene can also be applied to de-green citrus fruit or inhibiting potato sprouting. But in many cases, the presence of ethylene is undesirable. It limits storage time when fruit ripens too quickly and becomes overripe. This group includes products such as apple, apricot, avocado, kiwis, melon, papaya, peach, pear, plum, and tomato. Ethylene can also cause problems for products where ripening does not play a role. It can activate the senescence process (aging) in sensitive vegetables. Think of green beans, cabbage, lettuce, fresh cut herbs, broccoli, cauliflower, plant stock, potted plants and cut flowers. Ethylene can further cause or exacerbate some specific disorders in storage. Examples include superficial scald in apples, bitterness in carrots, death of buds in fruit trees, and flower abortion in tulip bulbs.
Whether ethylene leads to damage depends, among other things, on the commodity, temperature, ethylene concentration and exposure time. Ethylene production and ethylene sensitivity are not always related. Products that do not produce a large amount of ethylene themselves can be sensitive to ethylene and therefore suffer from external ethylene sources.

Sources of ethylene
In most cases, the source of ethylene is the fresh product itself. When ethylene in the room is above a certain limit value, this may indicate that the product ripens. Ethylene can also come from external sources such as industrial exhaust fumes, cars, gas forklifts, and adjoining rooms.
Practical solutions
Practical solutions to reduce negative ethylene effects focus on reducing ethylene production, reducing ethylene sensitivity and reducing the ethylene level in the air. The most common strategies to reduce negative ethylene effects are:
- First of all, cooling of most horticultural products is important. Low temperature helps to maintain good quality in many ways. One of the reasons is that a lower temperature can reduce both ethylene production and ethylene sensitivity.
- In Controlled Atmosphere (CA) and Modified Atmosphere (MA), reduced O2 and increased CO2 contribute to lower ethylene production and reduced ethylene sensitivity. The effect of ethylene is greatly reduced in Dynamic Controlled Atmosphere (DCA) storage of apples and pears with extra low O2 levels. A clear example is the ethylene related disorder ‘superficial scald’ in apple. The risk on this skin damage is clearly reduced in DCA storage.
- Avoid excessive ethylene from external sources. Ethylene can come from other commodities or external sources as mentioned in the previous paragraph. Keep these external sources away from the product. Store ethylene-sensitive and high ethylene-producing (ripe) products in separate rooms. Remove rotten products from the storage rooms.
- Ventilation during storage and transport (if no CA) can be an effective and relatively simple way to keep ethylene at a low level. Be sure to ventilate with air which is indeed ethylene-poor. We would like to stress here that the consequences of altered ventilation for other storage conditions, such as temperature and humidity, must be well thought out (see also: WUR postharvest consultancy, No. 3: How to influence water loss of horticultural produce in storage rooms). You must also realize that bulky plant organs such as fruit can still maintain a high internal ethylene content, even when ethylene is reduced in the environment. Strategies may be needed to reduce ethylene production.
- Ethylene removers or converters (scrubbers) remove ethylene from the air. This method can be based on potassium permanganate, via oxidation of ethylene. But there are various other commercial techniques. Ethylene removers can, for instance, be effective in commercial kiwi storage. Unripe kiwis produce low amounts of ethylene, but are very ethylene sensitive. For products that produce large quantities of ethylene, it can be a challenge to keep the ethylene content in the room sufficiently low.
- Inhibition of ethylene action can be achieved by applying ethylene antagonists such as STS (for ornamentals) and 1-MCP (for fruits, vegetables and ornamentals). These compounds work by inhibiting the ethylene effect in the products. Knowledge and measurements of ethylene are useful to optimize and understand the 1-MCP effect. Our experience is that the practical application can often be improved, for which we offer our services.

Collection and measurement of air samples from practice sites
We experience in our measurements at practice sites that ethylene-related problems are still very relevant. Measurements of ethylene shall be carried out accurately in order to draw valid conclusions. The appropriate practical measures can then be considered. Gas chromatography is a method of accurate measurement. Commercial ethylene analyzers using this method are also successfully used in practice.
Where such equipment is not available, air samples may be taken on site and transported to a research laboratory. We advise you to follow the correct procedures for this. An incorrect procedure for gas sampling in practice rooms can easily lead to wrong conclusions. Please note that the exact location and timing may affect ethylene concentration. Some influencing factors are the activity of ventilation systems and temporarily high concentrations caused by exhaust fumes from cars or gas forklifts. Depending on the situation, the most appropriate sampling equipment is available for each situation: syringes, inflatable bags, glass flasks, or special low-pressure canisters. Subsequent analyses of ethylene by GC shall be carried out using the appropriate method. Remember that reliable data is the basis for specific practical actions.

An example from practice: ethylene measurements
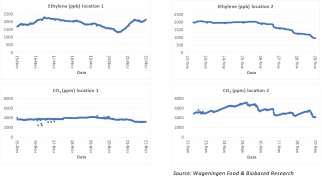
In the context of our advisory and research programme we examined the concerns expressed by a European wholesaler of fruit and vegetables. The company had expressed concerns about one of their distribution centers. We checked ethylene and CO2 concentrations at different sampling points for several weeks. The graphs below show the measured values of ethylene (ppb) and CO2 in the main hall (location 1) and in one of the storage rooms (location 2).
The graphs show a large fluctuation in both ethylene and CO2 over time. These fluctuations can be explained by the changes in commodities and volumes, the ripeness of the products, the storage conditions (temperature), and fluctuations in the air exchange by door openings or other ventilation. The ethylene measurements revealed values above 1000 ppb (1 ppm) and even above 2000 ppb (2 ppm). This level is significantly higher than recommended for ethylene-sensitive products. This can have a negative impact on quality. For this location, we have therefore advised to first check the air outlet of adjacent ripening rooms. The possible contamination of the distribution hall with ethylene-rich air from the ripening rooms can be prevented. A further strategy was to increase ventilation with fresh air.