
Longread
The many-sided role of carbon dioxide in postharvest practice
The role of carbon dioxide (CO2) in postharvest practice is very fascinating. A large amount of information about CO2 effects can be found in postharvest sources. The beneficial effects, but also symptoms of damage are well described. The purpose of the present article is to share some more background information and examples of CO2 action that are less well known, although very relevant to practice. As postharvest consultants of Wageningen Food & Biobased Research, we apply such scientific knowledge to improve the quality of horticultural products.
Relevance to practice
Elevated CO2, often together with reduced O2, is applied at various stages of the fresh supply chain. It is used for long-term storage (e.g. apples, pears, berries and kiwifruits), temporary storage or transport (e.g. strawberries, cherries and bananas) and modified atmosphere packaging of fresh-cut vegetables (e.g. lettuce). We experience many beneficial but unfortunately sometimes harmful effects of CO2 in practical situations. A better understanding of the CO2 mode of action helps to optimize its application for a better product quality.
Internal or external CO2
High CO2 can inhibit physiological processes, such as respiration and ethylene production. The concentrations of CO2 to achieve such inhibition are much higher than the approximately 0.04 % in the ambient air. As CO2 is a product of respiration, the CO2 exchange between the fresh product and its environment is important to take into account. The CO2 concentration inside bulky organs such as fruits and tubers, where the diffusion of gas is impeded, is often several % higher than the surrounding atmosphere.

This is especially the case at higher temperatures and advanced ripeness stages. Even when CO2 is kept low in the surrounding, CO2 can still be active due to its internal concentration. This can give a positive result, such as a longer shelf life. However, CO2 injury can occur in certain situations, even when ventilation or other measures effectively remove CO2 from the environment.
CO2 in aqueous solution
In postharvest we usually consider CO2 in its gaseous phase. It is good to realize that CO2 is soluble in water. This is very relevant for horticultural products with water as the main component. As with other gases, the solubility of CO2 in water increases as the temperature drops. Again relevant for horticultural products, with regard to cold storage temperatures.
When CO2 dissolves in water, some of it is hydrated to form carbonic acid (H2CO3). Due to the dissociation of carbonic acid to bicarbonate (HCO3-) and hydrogen ion (H+), pH of living cells could drop when exposed to high CO2. The intracellular pH values are normally regulated within narrow limits, but although not proven, acid stress may occur in fruits with high CO2 levels.
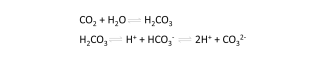
The reaction of CO2 with water can also be relevant on the exterior of cold products. When there is free moisture on the skin (e.g. condensation droplets) in an environment with high CO2, the carbonic acid can lead to an etched appearance of the fruit skin. Although very exceptional, this is a possibility to take into account when assessing damages of the fruit skin. This damage can be distinguished from a physiological damage. An important difference is that it is less dependent on variety, maturity or fruit size.

Inhibition or stimulation of ethylene production
CO2 is mostly associated with inhibition of ethylene production of fruits. But CO2 can also promote ethylene production rate, depending on the commodity and the CO2 concentration. It is known that CO2 can stimulate ethylene production by activation of the enzyme ACC oxidase. This stimulation has usually a short-term effect. At a relatively high (internal) CO2 concentration above 1% and/or prolonged exposure time, ethylene production is mostly reduced. This reduction is achieved by a different way of CO2 action.
A special effect of CO2: stomata in tulip bulbs
Sometimes remarkable CO2 effects are found that deserve an explanation. This certainly applies to the fact that CO2 can stimulate water loss of stored tulip bulbs. The actual mechanism of CO2 action on this water loss is unknown. But in one of the earlier studies within our advisory and research programme, we demonstrated that the most likely explanation involves the stomata which are present on the tulip bulb scales. While stomata in leaves tend to close when CO2 is increased (over low ranges), the situation for tulip bulbs may be quite different. We used scanning electron microscopy to study the stomata. It appeared that the stomata were blocked by a stomatal wax plug under normal gas conditions. When the bulbs were exposed to high CO2, the wax plug disappeared and the water loss increased. We also found a similar effect after exposure to ethylene and low O2.

Inhibition of mould growth
Harvested horticultural products may benefit from increased CO2 concentrations by suppressing mold growth. The minimum effective concentration to suppress Botrytis rot is 10% or even much higher. Of course, physiological injury to the products due to excessive CO2 should be prevented. When this happens, it can in turn even cause an increased incidence of decay.

Astringency in persimmon
Another special practice use of high CO2 is the de-astringency treatment. Some persimmon cultivars produce astringent fruit, caused by soluble tannins, and cannot be consumed as such. These fruit need a postharvest treatment to remove astringency by changing tannins into the insoluble form. High CO2 treatment (above 80%) is an effective method for astringency removal. The increase of acetaldehyde due to CO2-treatment provokes the polymerization of soluble tannin, which becomes insoluble. The fruits lose the astringent taste thanks to these high CO2 levels.
Conclusion
In the supply chain of fresh horticultural products, we strive for an optimal CO2 concentration. To optimize the use of CO2 we have to take into account the physiology of fresh products, the storage technique and the physical and chemical properties of CO2. In our consultancy work, we apply scientific knowledge to optimize CO2 conditions for a better product quality.